Maryland Department of Health (MDH) Laboratories Administration Guidelines and Instructions for Clostridium botulinum testing
General: CDC Botulism case definition and Guidance:
Botulism is a neuroparalytic illness characterized by symmetric, descending flaccid paralysis of motor and autonomic nerves, always beginning with the cranial nerves. Symptoms of botulism usually start with weakness of the muscles that control the eyes, face, mouth, and throat. This weakness may spread to the neck, arms, torso, and legs. Botulism also can weaken the muscles involved in breathing, which can lead to difficulty breathing and even death.
For more information: https://www.cdc.gov/botulism/health-professional.html
What testing can MDH Laboratories Administration Perform:
The Maryland MDH Laboratories Administration has the capability perform CDC developed standardized Laboratory Response Network (LRN) tests including bacterial cultures for Clostridium botulinum, real-time PCR ‘s targeting toxin genes, and toxin antigen detection/characterization assays for identifying cases infant botulism, adult botulism and for detecting the botulism toxin in suspected food products.
When can the MDH Laboratories Administration Conduct Testing for Botulism:
Botulism testing can only be conducted with prior authorization from MDH physician–epidemiologists.
Do not collect and submit specimens for botulism testing until an infectious disease consultation with the MDH physician-epidemiologist has been conducted and approval has been obtained to provide testing for the case.
What steps to take if you suspect a Botulism case:
To request botulism testing for a suspect case, contact the MDH Infectious Disease Bureau at 410-767-6700 during business hours and after hours the call Epidemiologist –on- call (443-827-2682) or the MDH Physician- on- call (443-717-4778) for the initial infectious disease consultation.
After the consultation if botulism testing has been authorized, if needed the MDH Laboratory will contact your institution’s laboratory to make any additional logistical arrangements for expedited testing.
Specimen Collection and Handling:
Guide to Public Health Laboratory Services (Page 47,48)
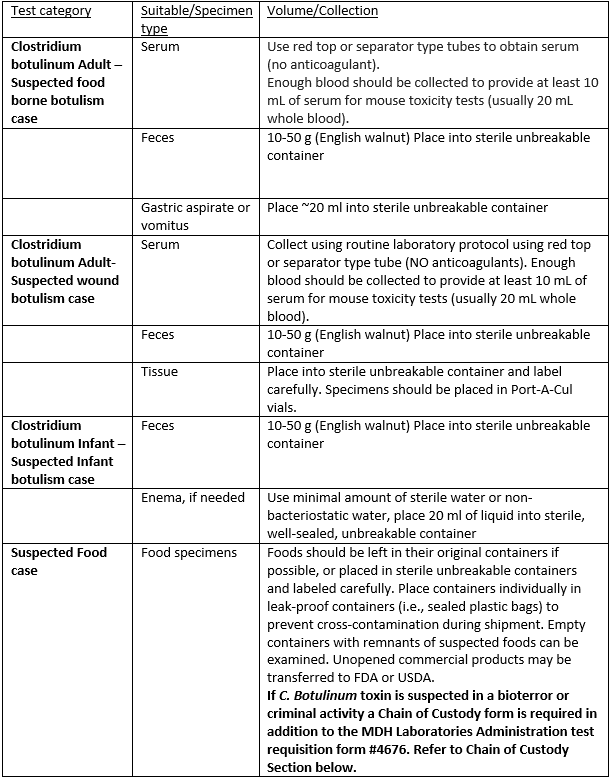
Notes:
- Submitting facility must have consent before sending specimen for testing. Contact the MDH Infectious Disease Bureau at 410-767-6700 during business hours and after hours the call Epidemiologist –on- call (443-827-2682) or the MDH Physician- on- call (443-717-4778) for the initial infectious disease consultation. This is considered a Public Health Emergency.
- Serum: Transport to the laboratory on wet ice or cold packs. If an unavoidable delay of several days is anticipated, the specimen should be kept frozen and then packed in an insulated container with dry ice and proper cushioning material for shipment.
- Stool: Transport to the laboratory on wet ice or cold packs. If an unavoidable delay of several days is anticipated, the specimen should be kept frozen and then packed in an insulated container with dry ice and proper cushioning material for shipment.
Test Request Form: The MDH Laboratories Administration Form # 4676 Infectious Agents: Culture / Detection must be completed and submitted with each specimen. Click on the links below for an example of the completed form #4676 and the electronic fillable form.
Packaging and Shipping
Specimens must be packaged in a triple packaging system to ensure that under normal conditions of transport they cannot break, be punctured or leak their contents (Refer to page 10 in the Lab Services Guide for Basic Triple Packaging Guidance).
Label container as follows
“MEDICAL EMERGENCY, BIOLOGICAL HAZARD, REFRIGERATE ON ARRIVAL”
Attention: LRN-B Laboratory

