High Consequence Novel Influenza A Testing at the Maryland Department of Health (MDH) Laboratories Administration
General Background
Influenza viruses are the causative agent of influenza infection, an acute highly contagious respiratory illness that each year results in high levels of morbidity and mortality. The Maryland Department of Health (MDH) Laboratory routinely conducts testing of seasonal influenza A and influenza B viruses from healthcare providers from across the State that are widely circulating in the United States. Statewide influenza monitoring by the Maryland Department of Health is important because it allows for a better understanding of viruses that are currently circulating in our communities, the existence of antiviral drug resistance and in rare instances identifies newly emerging influenza viruses that can lead to pandemics as was the case in with the emergence of the influenza A H1N1 virus in 2009. Additionally, since 2003 two avian influenza A viruses (H5N1) and (H7N1) have emerged in Asia that sporadically cause serve illnesses in patients with direct contact with poultry but to date have demonstrated limited sustained human to human transmissibility. These newly emerging avian viruses have the potential to be highly virulent and could evolve into strains that could be transmitted from human to human. As a result, the Maryland Department of Health has the ability to test for Influenza A/H5 (Asian lineage) and Influenza A/H7 (Eurasian Lineage) for travelers meeting the case definition who are returning from locations where these avian viruses circulate. For more information regarding novel avian influenza infection of humans please visit the CDC website: https://www.cdc.gov/flu/avianflu/
What testing can the MDH Laboratories Perform
The Maryland Department of Health (MDH) Laboratory has the capability to perform Influenza testing for influenza A subtypes A(H1)pdm09, A(H3), A(H5)(Asian lineage), and A(H7)(Eurasian lineage) and influenza B lineages B/Victoria and B/ Yamagata. Testing is performed using a CDC developed real-time PCR assay, with the detection of Influenza A (H7) being performed under Emergency Use Authorization (EUA). For more details of the performance characteristics of this assay refer to the Novel Influenza A Testing Interpretative Guidelines below. The MDH Laboratory can also run parallel tests for a variety of other common respiratory pathogens.
When can the MDH Laboratories Conduct testing for High Consequence (Novel) Influenza A(H5) and A(H7)
Influenza A(H5) and A(H7) testing can only be conducted with prior authorization from MDH physician–epidemiologists and is restricted to patients who meet the most current U.S. Department of Health and Human Services (DHHS) clinical and epidemiologic criteria for testing suspect A(H5) and A(H7) specimens.
WHO Case Definitions for Human Infections with Influenza A(H5N1) Virus
WHO Guidelines for Investigation of Human Cases of Avian Influenza A(H5N1) January 2007
Interim Guidance on Testing,Specimen Collection, and Processing for Patients with Suspected Infection with Novel Influenza A Viruses with the Potential to Cause Severe Disease in Humans
Do not collect and submit specimens for Influenza A(H5) and A(H7) testing until an infectious disease consultation with the MDH physician-epidemiologist has been conducted and approval has been obtained to provide testing for the case.
What steps to take if you suspect a Novel Influenza A Infection
To request High Consequence Influenza A testing for a suspect case of A(H5) or A(H7) contact the MDH Infectious Disease Bureau at 410-767-6700 during normal business hours. After hours contact the Epidemiologist on-call at 443-827-2682 or the MDH Physician on-call at 443-717-4778 for the initial infectious disease consultation.
After the consultation, if influenza testing is indicated and authorized, the MDH Laboratory will contact you or your institution’s laboratory to make any additional logistical arrangements needed for expedited testing.
Acceptable Specimens
Upper Respiratory Specimens
· Nasopharyngeal swabs (NPS)
· Nasal swabs (NS)
· Throat swabs (TS)
· Nasal aspirates (NA)
· Nasal washes (NW)
· Dual nasopharyngeal/throat swabs (NPS/TS)
Lower Respiratory Specimens
· Bronchoalveolar lavage (BAL)
· Bronchial wash (BW)
· Tracheal Aspirate (TA)
· Sputum
· Lung tissue
Specimen Collection Kit Instructions
For periods of ≤72 hours, specimens should be held at 2-8oC rather than frozen. For delays exceeding
72 hours, freeze specimens at -70oC as soon as possible after collection unless otherwise noted.
Please be sure to label each specimen with patient name, specimen type, and date and time of collection. Specimen collection kits (Viral Culture Kit) can be ordered by the local health department by calling 443-681-3777 and submitting via email or fax the Outfit Supply Requisition form. (See link below)
Outfit Supply Requisition 2019 Fillable Form 1019
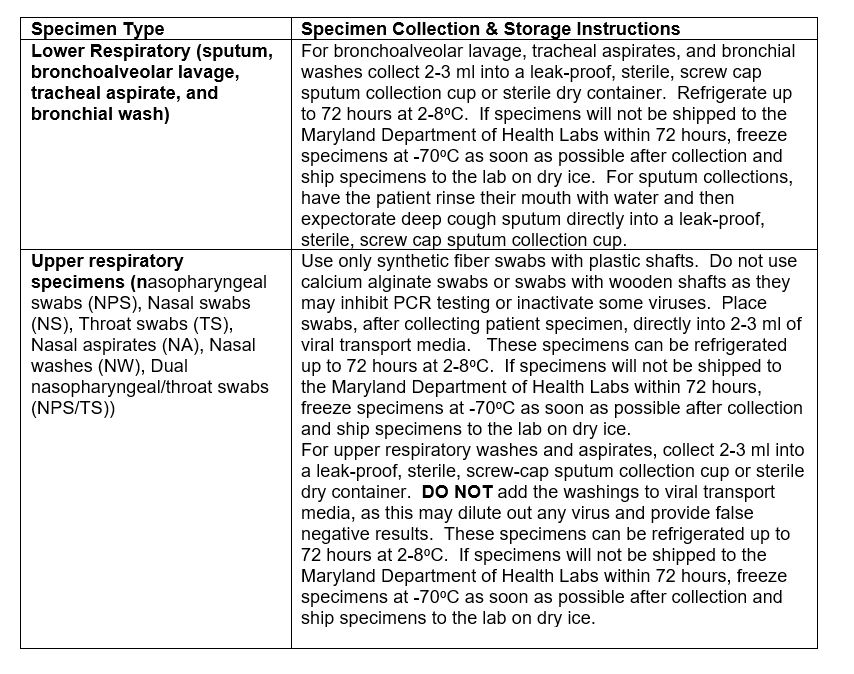
Specimen Collection & Storage
Completing the Test Request Form
The MDH Laboratories Administration Form # 4676 Infectious Agents: Culture / Detection must be completed and submitted with each specimen. Click on the links below for an example of the completed form #4676 and the electronic fillable form.
Requisition Sample Completed Form
Fillable MDH 4676 Infectious Agents LaboratoryRequisition
Packaging and Shipping
Specimens must be packaged in a triple packaging system to ensure that under normal conditions of transport they cannot break, be punctured or leak their contents (Refer to page 10 in the Lab Services Guide),
Packaging and Shipping Guide (Pages 9 to 11)
https://www.cdc.gov/labtraining/training-courses/packing-shipping-division-6.2-materials.html
Contact Information:
To request Novel Influenza A testing contact the MDH Infectious Disease Bureau at 410-767-6700 during normal business hours. After hours contact the Epidemiologist on-call at 443-827-2682 or the MDH Physician on-call at 443-717-4778 for the initial infectious disease consultation.
For questions or concerns, please contact the Division of Molecular Biology Laboratory at 443-681-3924 or 443-681-3800 during normal business hours from 8:00AM-4:30PM.
Novel Influenza A Testing Interpretative Guidelines
FACT SHEET FOR HEALTHCARE PROVIDERS: INTERPRETING CDC HUMAN INFLUENZA VIRUS REAL-TIME RT-PCR DIAGNOSTIC PANELINFLUENZA A(H7) [EURASIAN LINEAGE] ASSAY TEST RESULTS
FACT SHEET FOR PATIENTS: UNDERSTANDING RESULTS FROM THE CDC HUMAN INFLUENZA VIRUS REAL-TIME RTPCR DIAGNOSTIC PANEL–INFLUENZA A(H7) [EURASIAN LINEAGE] ASSAY

